
Spectroscopic systems used to create a laser
Not all atoms, ions and molecules, with their different energy levels, are capable of creating a population inversion and a laser effect. Only radiative transitions (where the atoms are excited due to light absorption) should be used and non-radiative transitions should be avoided. Some transitions have both a radiative and a non-radiative part. In this case, the upper level empties as a result of a non-radiative effect as well as spontaneous emission. This leads to additional problems for achieving a population inversion because it is difficult to store atoms in the upper level under these conditions. Thus, this type of transition should also be avoided.
Next, the relative energy levels specific to each type of atom must be considered. For example, choosing a lower level with more energy than the ground state will greatly limit the population N1, which may even be zero (Figure 3). This means that only one atom would have to be excited to achieve population inversion.

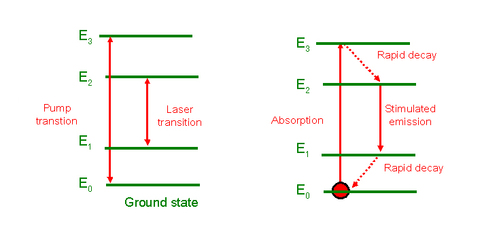
In addition, pumping must be able to move atoms to a higher level. Every pumping system (particularly optical or electrical) corresponds to a certain energy, which must be transferable to the atoms of the medium. The difference in energy between the excited state and the ground state must match the pumping energy. In optical pumping, there must be at least three different energy levels to create a population inversion. Figure 4 illustrates such a system. It shows the pumping transition (between E1 and E3) and the laser transition (between E2 and E1). The objective is to store atoms in level E2 by absorbing “pumping” radiation whose wavelength is shorter than that of the laser transition. This means that the excited atoms must quickly decay from level 3 to level 2 only, a condition that limits the choice of systems that will work. Figure 4 also shows an ideal cycle for an atom: it rises into level 3 by absorbing a photon from the pumping light. It then falls very rapidly into level 2. Finally, it decays by stimulated emission to level 1. Despite its simplicity, this is not a very easy system to implement as the ground state of the laser transition has a large population at thermodynamic equilibrium and at least half of this population must be excited to level 2 to obtain population inversion. Moreover, level 2 must be able to store these atoms so spontaneous emission must be very unlikely. This affects the choice of the system. A large pumping energy is also needed. The first ever laser was of this type and used a ruby (Cr3+:Al2O3). Ruby is composed of an aluminium crystal matrix and a doped ion (Cr3+) whose energy levels are used to create the laser effect. The medium is strongly pumped by discharge lamps.

Another example of a spectroscopic system is the four-level laser (Figure 5). Here, the pumping transition (optical pumping) and the laser transition occur over a pair of distinct levels (E0 to E3 for the pump and E1 to E2 for the laser). E1 is chosen to be sufficiently far from the ground state E0 so that the thermal population at thermodynamic equilibrium is negligible. Similarly, atoms do not stay in level 3 or level 1. Figure 5 represents an ideal four-level system. Unlike the three-level system, as soon as one atom moves to level 2, a population inversion occurs and the medium becomes amplifying. To maintain the population inversion, atoms must not accumulate in level 1 but must rapidly decay to level 0. One of the best known mediums operating in this way is neodymium YAG (Nd3+:Y3Al5O12).
A final example of a spectroscopic system providing a laser effect is the helium-neon gas system (Figure 6). In this case the pumping method is electrical. Neon transitions are used for the laser transitions: there are several but the most well-known is the coloured one at 632.8 nm. Helium is used as an intermediary gas, capable of transferring energy from the electrons to the neon particles via collisions. Helium is also unique in having two excited states said to be “metastable” i.e. atoms can stay there a long time before falling to the ground state. Helium atoms are carried into the excited state by collisions with electrons. Energy is easily transferred to neon when the atoms collide because these metastable levels coincide with the excited states of neon. This process is given by the equation:
He* + Ne -> He +Ne*
An excited helium atom meets a ground-state neon atom and transfers its energy while decaying.
Figure 6 also shows that the lower levels of the laser transitions are far from the ground state, which favours population inversion (no thermal population).
