
Presentation of the energy levels
The neodymium ion (Nd3+) in the YAG matrix (Y3Al5O12, yttrium aluminium garnet) has a great many levels that can give different laser transitions. Figure E1 locates the energy levels by wavenumber
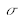 expressed by convention in cm-1. The wavelength
expressed by convention in cm-1. The wavelength
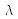 (in m) corresponding to a transition between levels 1 and 2 is obtained from:
(in m) corresponding to a transition between levels 1 and 2 is obtained from:
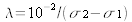 .
.
The energy levels of the neodymium ion Nd3+ are represented by a group of letters and numbers that give the quantum numbers associated with different components: the letter corresponds to the orbital quantum number, the superscript number gives the spin quantum number and the subscript fraction is the angular quantum number. Because of the crystal field (Stark effect), the energy levels are split into sublevels, represented by letters with subscripts (Z1...R2).
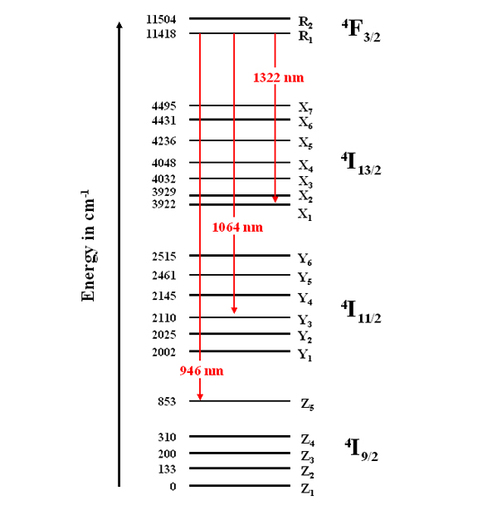
The neodymium ions only stay a long time in the level 4F3/2. The lifetime of this level is around 230 μs while it is less than a nanosecond in the other levels. Thus, the ions accumulate in this level and can fall from it by intense stimulated emission.
The lifetime of an atom in a level is the average time it is present in this level before de-excitation. It can be shown that, if the population of the level is N0 at t=0 s, then
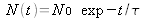 , at the instant t, where
, at the instant t, where
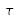 is the lifetime.
is the lifetime.
Figure E1 shows a great many levels and thus numerous possibilities for laser emissions and transitions from level 4F3/2. The red arrows give the wavelengths of the most used laser transitions: 1064 nm corresponds to the transition with the greatest probability of stimulated emission. There is also a line in the further infrared towards 1320 nm. Finally, the Nd:YAG has another quite efficient transition in the near infrared at 946 nm.