
Energy Bands
Consider an isolated silicon atom; its energy levels are quantized (see the Bohr model for Hydrogen). When two identical atoms are brought closer together, the quantized energy levels hybridize and split into two different levels because of the mutual interaction of the two atoms. More generally, when N atoms are moved closer, until they reach the equilibrium inter-atomic distance d, the energy levels split into N levels. These N levels are very close to each other if N is large (which is the case in a crystal) so that they eventually form a continuous energy band.
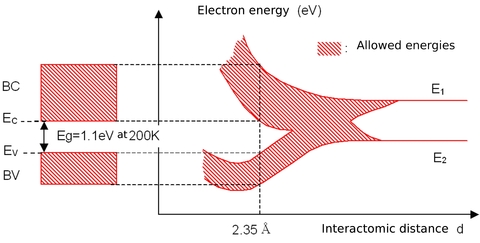
Let's now consider silicon atoms arranged in a periodic lattice, but with a very large lattice parameter (or inter-atomic distance), in order to first consider each atom as isolated. The two levels with the highest energy are labeled E1 and E2. Now let's shrink homothetically the atom lattice: energy levels split and form two continuous bands known as the conduction band CB and the valence band VB, Figure 1 shows the formation of these bands as a function of the inter-atomic distance.
In a silicon crystal (
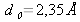 ), two continuous energy bands exist (CB and VB), separated by a forbidden band, which is not accessible for electrons. This forbidden region is called the « gap » and its width Eg is a characteristic of the material. The lowest energy level of the conduction band is denoted EC and the highest energy level of the valence band is called EV so that we have the relationship Eg=EC-EV. The conduction and valence bands CB and VB represent the energies accessible to electrons, or the energies of the states potentially occupied by electrons: they do not provide any information about the effective occupation of the energy states by electrons.
), two continuous energy bands exist (CB and VB), separated by a forbidden band, which is not accessible for electrons. This forbidden region is called the « gap » and its width Eg is a characteristic of the material. The lowest energy level of the conduction band is denoted EC and the highest energy level of the valence band is called EV so that we have the relationship Eg=EC-EV. The conduction and valence bands CB and VB represent the energies accessible to electrons, or the energies of the states potentially occupied by electrons: they do not provide any information about the effective occupation of the energy states by electrons.